The second general area of interest in the lab is to understand how timing of developmental processes is regulated. In many organisms systemically delivered steroid hormones modulate developmental transitions, including metamorphosis in insects and puberty in humans. Our previous work has identified the neuroendocrine signals that trigger steroid production and release in Drosophila melanogaster (Shimell et al. 2018; Yamanaka et al.2015), as well as characterized the cell biology of steroid production and secretion in endocrine cells and determined how biosynthetic intermediates are trafficked between the mitochondria and the ER. More recently, we have characterized inputs into the critical weight checkpoint and their effects on developmental timing and body size (Shimell and O’Connor 2023, Pan and O’Connor 2021, Pan et al. 2019)
We are currently looking into the mechanism responsible for a prolonged development program in Drosophila harboring a mutation in the Trus gene and examining the subcellular localization of steroid biosynthetic enzymes.
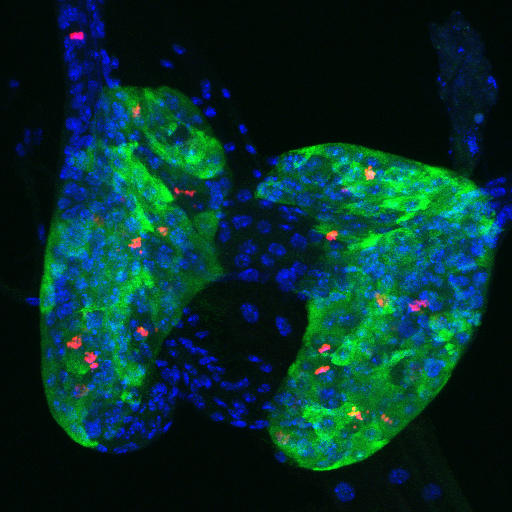
Endoreplication in the major larval endocrine organ is dispensable for the critical weight checkpoint.
Prothoracic gland (PG) of a wandering third instar larva expressing Fzr (Fizzy Related)-RNAi in the cells of the PG. The fzr gene is needed for the mitotic-to-endocycle switch during the previous instar stage. Thus, in wild type Drosophila, PG cells are normally endoreplicating at this point. In this case, PG cells continue mitotically dividing, as shown by red staining to anti-Phospho-Histone 3 antibody, resulting in a larger PG containing more cells. These larvae achieve the normal critical weight, undergo metamorphosis and yield viable adults. The anti-spookier antibody (green) is specific to PG cells and DAPI (blue) stains the nuclei.
From Shimell and O'Connor MicroPubl Biology, 2023. Image by MB O'Connor.

Trus is implicated in ribosomal function, cell cycle regulation, and cell proliferation.
On the left is a wild type Drosophila larval brain, ventral nerve cord, and imaginal discs at the wandering stage, about 4 days after egg lay. Note overall size and number of cells. Mitotically dividing cells are stained green with anti-phosphoHistone 3 antibody. On the right is a Trus mutant brain, ventral nerve cord, and imaginal discs also at the larval wandering stage, but about 10 days after egg lay. Image from S. Takada
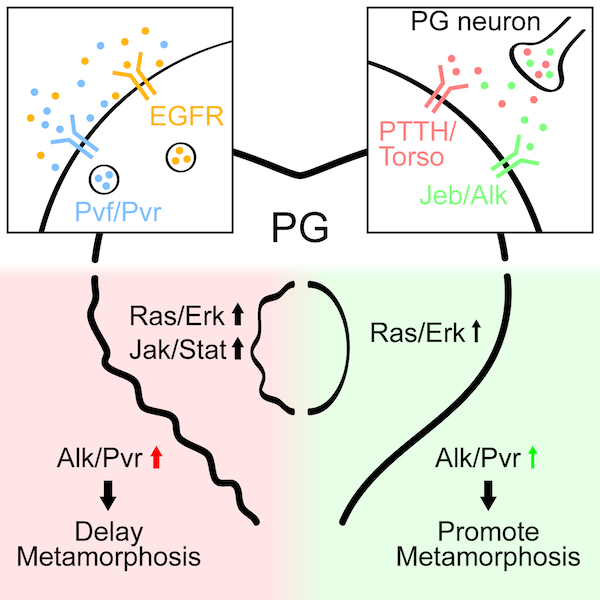
Additional signaling pathways that control developmental timing and body size in Drosophila
Pan and O’Connor identify Jeb/Alk and Pvf/Pvr as additional signaling pathways that control developmental timing and body size in Drosophila. Jeb/Alk signaling occurs in a juxtracrine fashion between the prothoracic gland (PG) neurons and the PG to control ecdysone production, while Pvf/Pvr signals via autocrine activity within the PG itself.